
Australia’s most read investor-focused magazine for ASX-listed Tech & Biotech stocks
Trending Stories

24 January, 2026
Drones, Jammers and Valuations: Bell Potter Declares 2026 the Year of the UAV
If there was still any doubt that drones had reshaped the battlefield - and the boardroom - Bell Potter Securities’ Drone Report puts it to rest. Published on 21 January 2026 by analysts Baxter Kirk and Ritesh Varma, the 9-page report lays out the bullish case for unmanned aerial systems (UAS) and their countermeasures (C-UAS), positioning the ASX as a surprisingly rich source of exposure.
Driven by new legislation, surging defence budgets and real-time battlefield feedback - most visibly in Ukraine - Bell Potter sees 2026 as an inflection year for drone and counter-drone markets globally. Drones now account for 70-80% of daily combat losses in the Ukrainian conflict. That has tipped procurement scales from future-looking experiments to urgent acquisition programs.
The C-UAS Playbook
The legislative lever, at least in the US, has been firmly pulled. The Safer Skies Act, passed in December 2025, permits public safety agencies - including law enforcement and correctional facilities - to disable drones deemed a credible threat. This is a structural change. For the first time, state and local governments can procure and deploy C-UAS tools, unlocking an estimated 12% of the addressable market for non-kinetic countermeasures such as RF jammers.

DroneShield RFPatrol Mk2 (supplied)
DroneShield (ASX:DRO)’s DroneGun Mk4 and RFPatrol Mk2 units are already deployed in such scenarios. In fact, the report notes the US Air Force continues to single out DroneShield’s gear as the only proven option that meets its handheld requirements.
Directed energy weapons (DEWs) are another area of focus, where Electro Optic Systems (ASX:EOS) is gaining traction, having recently delivered its 100kW “Apollo” system into Dutch and Korean defence programs. Europe, meanwhile, is forging ahead with its “Drone Defence Initiative,” with Poland’s €2.5 billion “East Shield” and the Netherlands both identified as early movers.
Amid this surge, the value of adaptable, component-based supply chains has become clear. Bell Potter doesn’t highlight unlisted suppliers, but it notes that scalable defence engineering is increasingly a function of distributed, low-capex manufacturing. That’s a subtle nod to firms like KTEK Systems, a Tier-2 provider of UAV sub-assemblies and ruggedised airframes, which operates under an asset-light “Cordless Factory” model across Israel, the EU, and soon Australia.
Drones in Demand - Fast
On the offensive side of the ledger, the US Department of War is now targeting production of 340,000 small drones over two years, with unit prices capped at around US$5,000. This high-volume, low-cost procurement plan favours agile manufacturers - especially those already embedded in Tier-1 OEM programs.
KTEK, for instance, is a known supplier to UVision, whose Hero-120 loitering munition secured a US$982 million multi-year IDIQ contract from the US Army in October 2025. KTEK produces the composite fuselage and sub-systems for that platform - a position that could provide tailwinds without requiring direct contract attribution.
That’s the model. Support the primes, stay in the background, and scale as they scale.
Commercial Flight Path Also Clears
It’s not all battlefield firepower. The commercial drone market is quietly gearing up for a breakout, pending the long-awaited FAA Part 108 reform, which is expected to permit scalable Beyond Visual Line of Sight (BVLOS) operations. Bell Potter flags this as a potential game-changer for Elsight (ASX:ELS), whose Halo platform has already been validated for public safety use.

The report also notes the inflection point at Australian mine sites, where autonomous deployments are growing - RocketDNA (not rated) is rolling out over 20 xBot units. It’s another sign that the demand curve for smart UAV systems is steepening not just for platforms, but for the electromechanical and connectivity subsystems beneath them.
KTEK, which also supports ISR and C4I ground infrastructure projects via ruggedised racks, enclosures, and power distribution modules, stands quietly aligned with these commercial and dual-use trends.
Valuations and Market Positioning
One of the more compelling parts of the Bell Potter analysis lies in its relative valuation work. It compares ASX-listed drone and counter-drone companies with global peers like AeroVironment (NASDAQ:AVAV) and Kratos (KTOS), and finds a significant multiple discount. ASX names like DRO, ELS and EOS are trading at sub-30x FY28 EV/EBITDA, while their US peers are fetching multiples of 50x or more.
This pricing gap, in Bell Potter’s view, may present an opportunity - particularly for investors looking for exposure to a sector on the verge of structural expansion. The research note rates DRO, ELS, and EOS as “Buy” and flags a catalyst-rich six months ahead.
That optimism may also extend to IPO candidates orbiting the listed space. KTEK, for instance, has already completed an oversubscribed seed round backed by Regal Emerging Companies Fund and is planning an ASX listing later this year.
Risks Still Apply
Despite the tailwinds, Bell Potter isn’t blind to risk. The report highlights execution, regulatory approvals, R&D competitiveness and geopolitical stability as key pressure points. It also underscores the importance of diversifying revenue bases - an area where some ASX players still lean heavily on single customer relationships.
That said, for companies with proven capability, scalable production models, and Tier-1 integration credentials, the risk-reward trade-off in 2026 appears increasingly skewed to the upside.
Where It Leaves Investors
If The Drone Report is correct, drones are moving from the tactical edge to the strategic core of global defence policy - and the ASX is well positioned to ride the wave. Investors eyeing the sector would be wise to look not only at the headline names, but also at the specialised ecosystem forming beneath them.
Because in the new world of autonomous systems, the companies building the hardware, racks, and ruggedised frames - quietly, reliably - might just end up doing the heavy lifting.
READ ARTICLE
21 January, 2026
Audeara’s Hearing Health Push Hits High Notes in Asia and Australia
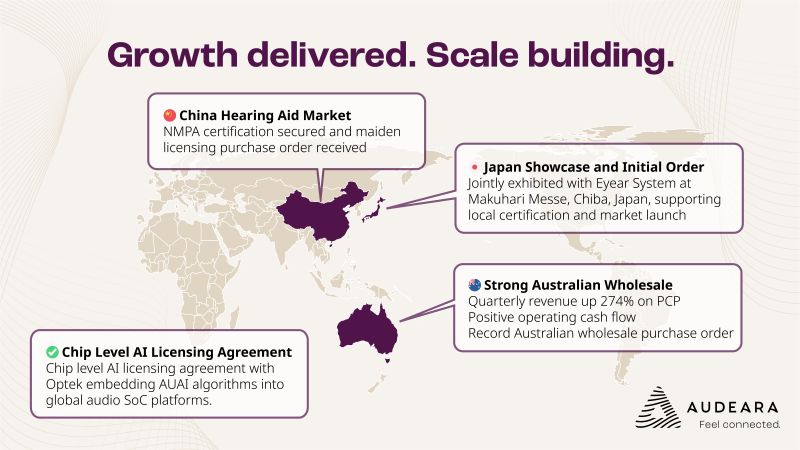
ASX-listed hearing tech specialist Audeara (ASX: AUA) is striking all the right chords as it rounds out the first half of FY26, with surging revenues, fresh licensing deals, and a growing global footprint that has the company closing in on last year’s full-year sales haul in just six months.
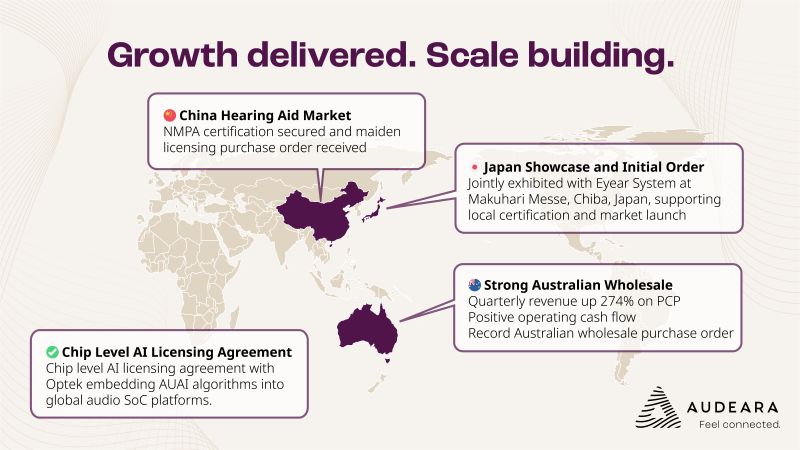
Group revenue for Q2 FY26 came in at $1.43 million – flat quarter-on-quarter, but up a resounding 274% on the prior corresponding period. For the first half of the financial year, combined with a chunky $560k wholesale purchase order to be recognised in Q3, the company is already within spitting distance of its FY25 revenue of $3.8 million.
And while some small caps dream of operational cash flow breakeven, Audeara posted a $356,000 positive operating cash result for the quarter, buoyed by a $1.22 million R&D tax refund.
A ‘Hear’ and Now Opportunity in China
Arguably the most ear-catching news came out of China, where the company received its maiden licensing order from Eastech Co. Ltd, and more critically, cleared the last regulatory hurdle with certification from China’s National Medical Products Administration (NMPA). This opens the door to full commercial launch of its hearing aid technology into the vast Chinese market, via a local partner and under an established third-party brand.
This is no small beer. With manufacturing already underway and the product set to be distributed through China's dominant online health and retail platforms, the model is low capital intensity and high scalability – precisely what investors want to hear.
Australia Holding Its Own

Photo supplied by Audeara
\Back on home turf, the Australian wholesale business continues to hum along nicely. In fact, Audeara secured its largest ever local order in late November – a $560,000 commitment from a major customer. Once delivered in February and booked in Q3, that single deal will take FY26 wholesale revenue to $1.83 million – matching the entirety of FY25 and representing a 58% increase on the prior year half.
Licensing in Stereo: Japan, Taiwan, and Beyond
The company’s ambitions are firmly tuned to global markets. In Japan, a distribution agreement was inked with Eyear System Inc., setting the stage for its Auracast™-enabled products to enter one of Asia’s most advanced audiology markets. The two parties also showcased their wares at the Makuhari Messe event in Chiba – Japan’s healthcare tech mecca.

Photo supplied by Audeara
Meanwhile in Taiwan, Audeara’s partnership with Clinico Inc. bore fruit, with the jointly developed CS1 hearing buds snagging the SNQ National Quality Mark – a key stamp of approval as the product rolls out into retail channels.
Embedded Tech: AI on Chip
On the tech front, a licensing deal with Optek Microelectronics could prove to be a sleeper hit. The agreement allows Audeara’s AI-powered audio algorithms to be embedded at the chip level in Optek’s systems – think fee-per-chip royalties across major electronics brands. While not yet revenue generating, this agreement has the potential to open the floodgates, given Optek’s impressive client list (Sharp, Panasonic, Philips, Toshiba et al).
Crunching the Numbers
Aside from the standout revenue growth, the numbers tell a story of cautious momentum: operating cash receipts were $732k for the quarter, with $960k in receivables and pending orders due to land shortly. Cash at bank was $737k, down from $1.2m, primarily due to loan repayments following the R&D rebate. Related party payments were a steady $133k, with minimal capex outlay and modest financing costs.
For a company that began life selling headphones tailored for audiology, Audeara is now amplifying its presence on the global hearing stage. With a tech stack that’s resonating across continents and a revenue profile that’s becoming more balanced and resilient, it’s safe to say investors will be watching – and listening – closely as the second half of FY26 plays out.
Outlook: Sounding Off on the Second Half
CEO Dr James Fielding summed it up well: “The business is demonstrating tangible scale and consistency across both our wholesale and technology licensing channels. This momentum positions Audeara strongly as we move into the second half of FY26”.
The company’s to-do list for H2 includes converting development partnerships into recurring revenue, ramping China sales post-certification, and expanding Auracast distribution – all while keeping the cost base under control. If the first half of FY26 is anything to go by, the company is certainly not tone-deaf to the opportunities ahead.
READ ARTICLE

20 January, 2026
Pointerra’s Q2 FY26: Cash-Flow Positive and Digital Twin-ning All the Way
Perth-based Pointerra (ASX:3DP) has delivered a December quarterly that's as cloud-based and analytics-driven as its 3D digital twin platform suggests - but also pleasantly grounded in that rarest of tech startup beasts: positive cashflow.
In its Q2 FY26 Appendix 4C and accompanying investor presentation released on 21 January, Pointerra posted customer receipts of A$2.61 million, a tidy 30% bump on Q1’s $2 million, and nearly double the $2.4 million generated across the entire second half of FY25. Net operating cashflow came in at a positive $472,000, reversing a $336,000 outflow in the prior quarter, and leaving the company with $2 million in the bank at year-end.
This upswing wasn’t merely a product of one-off wins - though some chunky milestones certainly helped. Pointerra has started booking revenue under a US$2 million contract with Georgia Power as part of the U.S. Department of Energy’s GRACI program. The project, focused on grid resilience, marks a new partnership with global energy consultants Baringa, and will contribute material revenue through calendar 2026.
Cloud Clout: Sector Traction Grows
Pointerra's digital twin platform, now sold under a tiered model of Core, Analytics, and Answers, continues to embed itself deeper into industries where physical asset management is no longer just about clipboards and CAD files. The company reported commercial wins and deeper integrations across its five key sectors - energy, mining, AECO (architecture, engineering, construction and operations), transport, and government.

The standout growth continues to come from the US energy utility market, where Pointerra nabbed a multi-year platform commitment from a major West Coast utility. This customer, notably, has implemented the Teledyne Optech Galaxy onboard bundle - the first such real-world deployment since the companies announced their partnership in early 2025.

Meanwhile, in the mining and oil & gas sector, a major hazard management pilot with a Tier 1 miner has gained momentum and now includes closure management tools - hinting at eventual enterprise rollout. Similarly, a proof-of-concept with a Tier 1 oil and gas operator concluded successfully, and contract discussions are underway to integrate Pointerra3D into the operator’s day-to-day inspection workflows.
Survey Says: Digital Twin Tech Hits the Masses
Not content with merely hunting Tier 1 whales, Pointerra is also cultivating a long-tail customer base through its Digital Surveyor package, targeting small UAV operators and surveyors. The model, coupled with its photogrammetry engine and new consumption-based pricing - where users buy Processing Units à la AWS - is finding traction. The company rightly sees this as a scalable annuity-style revenue stream as the sector continues to digitise.

In the AECO space, the recently completed pilot with Amazon - covering multiple sites across the US and UK - validated Pointerra’s ability to scale across a global logistics footprint. Amazon has now approved the Perth outfit as a direct supplier, smoothing the way for broader rollout in 2026 and beyond.
Back on home soil, Transport for NSW and Sydney Metro are exploring expanded use of Pointerra3D to manage tunnel inspections, station construction and broader digital asset management. Across the pond, various US State Departments of Transportation are trialling the platform too, highlighting growing appeal in the transport infrastructure sector.
R&D: New Data Models for a Multi-Modal World
Behind the scenes, the platform is evolving. A foundational overhaul of Pointerra3D’s data architecture is underway to better support complex, multi-modal datasets beyond point clouds - including time-based projects and more scalable automation. Additions like 360-degree video support and AI-assisted in-app help also hint at a UX glow-up that’s more Apple than Autodesk.
More functionally, the team is sharpening its edge in lidar-based vegetation analytics for utilities, streamlining orthoimagery workflows for drone and satellite data, and beefing up support for large unstructured 3D meshes - the sort often found in mining and terrain models.
Profitability on the Horizon?
While the path to sustainable profitability has previously felt more aspirational than assured, this quarter marks a concrete step forward. Management notes that positive cashflows are expected to continue in Q3, underpinned by over $2.5 million in receivables and contracted work at the end of December.
Pointerra’s software-as-a-service model, heavy on recurring revenue and low on physical infrastructure, is starting to show the leverage one expects from scale. Whether this momentum carries through into meaningful EBIT margin territory remains to be seen, but for now, the company has made its case: it’s not just building digital twins - it’s building a viable, cloud-based business to go with them.
READ ARTICLE

20 January, 2026
Mantis Goes Global: FBR’s Robotic Welding Arm Snags $990k US Order
Perth-based robotic technology company FBR Limited (ASX: FBR) has marked a major step in the commercial rollout of its Mantis® welding robot, announcing a $990,000 conditional sale to US-based industrial equipment dealer State Machinery & Equipment Sales. The deal represents Mantis’s first overseas order and a major endorsement of FBR’s strategy to expand beyond its better-known Hadrian® bricklaying system.

A Prototype with a Purchase Order
Though Mantis remains in its prototyping phase, State Machinery has placed a binding conditional purchase order - a clear vote of confidence in the robot’s potential. The Louisiana-based firm plans to deploy the unit in the manufacture of hopper barges at its Mississippi River facility. That’s no small task: barge construction requires precise, heavy-duty welding and high reliability, all of which Mantis promises to deliver.
But before any sparks fly in the US, Mantis must prove its chops back home. The purchase hinges on a successful Factory Acceptance Test (FAT) conducted at FBR’s Western Australian headquarters.
The FAT Test: A Trial by Fire (and Steel)
To secure the first $450,000 instalment of the contract, Mantis must weld a sub-assembly of a barge to rigorous technical standards. These include:
A rapid traverse speed exceeding 10 metres per minute
A linear weld speed of over 300 millimetres per minute
Weld quality that passes non-destructive testing, per AWS D1.1 structural steel welding codes
Third-party inspectors will be brought in to verify the results. Should Mantis hit those marks, the remaining payments - $450,000 upon delivery (expected in the second half of calendar 2026) and $90,000 three months later - will follow. FBR is also responsible for installation and training on site in Louisiana.
High Hopes for High-Speed Welding
FBR CEO Mark Pivac is bullish on the Mantis’s performance capabilities, claiming it could surpass the FAT targets by a factor of four. “We have agreed the FAT welding speed based on AWS pre-qualified welding speeds,” he noted. “Our Mantis high deposition welding should be able to weld over four times faster, and we look forward to demonstrating that”.

This isn't mere marketing bravado. If Mantis performs as expected, it could disrupt heavy fabrication sectors like shipbuilding, defence manufacturing, and mining - industries where scale, safety, and consistency trump low-cost labour.
US Partner Sees Big Potential
State Machinery’s endorsement of Mantis carries weight. The company is a prominent dealer of construction and manufacturing equipment in the southern US, and has experience evaluating high-capacity machinery. President Ed Renton said the firm was drawn to FBR’s robotics after observing the capabilities of Hadrian®.
“We are very excited to get our hands on the first Mantis® in the world,” Renton said. “We are pleased to be working with the team to bring their robotic welding technology to the United States to boost our manufacturing capability”.
Diversifying Beyond Bricklaying
FBR’s reputation was built on Hadrian®, its bricklaying robot now used to deliver “Wall as a Service®” to residential builders. But Mantis marks a strategic shift into new verticals. Both systems run on FBR’s proprietary Dynamic Stabilisation Technology® (DST®), which allows precise robotic control in outdoor environments.
By targeting heavy welding jobs, FBR aims to expand its relevance in industrial sectors that have historically resisted automation due to complexity and variable conditions. Mantis could help bridge that gap.
The company has long been seen as a one-product story, but the Mantis order gives substance to its diversification strategy. Although the FAT still looms large as the next hurdle, the contract’s size and structure suggest this is more than a speculative toe-dip. It's a potential revenue stream with international legs - and, if successful, could open the door to other markets facing similar manufacturing bottlenecks.
While it's early days, this deal offers investors a rare concrete milestone. It's not a revenue promise years in the making, nor a speculative partnership. It's a real (albeit conditional) contract with staged payments, performance criteria, and a delivery date. The long lead time means cash won’t hit the books until late 2026, but for a company looking to convert R&D into revenue, the signal is clear: Mantis is more than just a lab project.
And it’s worth noting the strategic implications. This could be the start of a new leg of growth for FBR - one that sits alongside Hadrian but opens access to different industries, customers, and geographies. The Mississippi might just be the beginning.
READ ARTICLE

14 January, 2026
A Green Giant Emerges: LGP and Cannatrek Tie the Knot in a $112m Cannabis Merger
In a move set to reshape the Australian medicinal cannabis landscape - and shake up the European scene while they’re at it - Little Green Pharma (ASX: LGP) has announced a transformational merger with unlisted rival Cannatrek, creating a vertically integrated juggernaut with global aspirations.
Under the scheme implementation deed signed on 14 January 2026, LGP will acquire 100% of Cannatrek’s issued capital via a scheme of arrangement. Post-merger, Cannatrek shareholders will emerge with 60.5% of the combined entity, with the potential to scale up to 68.2% depending on post-completion adjustments. LGP shareholders will be diluted to 39.5%, potentially dropping to 31.8%.
The Marriage of Muscle and Maturity
Both parties bring substantial firepower to the table. Cannatrek, headquartered in Melbourne, is one of the country’s largest and most successful cannabis firms, boasting a full-service model spanning cultivation, GMP-certified manufacturing, digital health platforms, and the country’s top-selling medicinal cannabis flower.

Cannatrek cultivation facility in Victoria, Australia
Meanwhile, LGP’s European footprint is formidable. It owns the largest cannabis production facility in Europe - in Denmark - and sells into more than a dozen export markets. While it’s a leader in Australia, LGP has shown notable foresight in staking a claim in the EU well before the current continental green rush took off.

Little Green Pharma Danish production facility
If you simply mashed their FY25 numbers together, you’d get a group with $112 million in revenue, $13 million in adjusted EBITDA, and a war chest of $15 million in cash. Importantly, they’d be sitting on net assets north of $136 million - a solid base from which to build a global brand.
Driving Scale in a Crowded Market
The merger is less about love and more about logic. In a market where operational scale, brand recognition, and deep distribution pipelines are becoming prerequisites for survival, the deal ticks all the strategic boxes. LGP's MD Paul Long said the deal would ensure the combined group “emerges as one of the largest and most vertically integrated medicinal cannabis businesses globally and a leading player in the Australian and European markets”.
Cannatrek chair Brent Dennison struck a similarly bullish tone: “We expect that by joining forces, we can enhance the profitability profile of the Combined Group in Australia with an ongoing emphasis on strong cash generation”.
Indeed, the synergy bingo card is almost fully marked: LGP’s excess capacity in Denmark can backfill Cannatrek's export needs, while Cannatrek’s Australian GMP capabilities can alleviate pressure on LGP’s domestic ops. Cost efficiencies are expected through consolidated clinic operations, optimised expense management, and a bolstered product suite of eight brands.
Contingent Value Shares: The Deal Sweetener
To address potential unknowns, Cannatrek shareholders will also receive contingent value (CV) shares - 0.727502 for every Cannatrek share held. These will convert into LGP shares in two years based on a balancing act of post-merger liabilities. If Cannatrek’s liabilities outweigh LGP’s by more than $2 million, fewer CV shares convert; if the reverse is true, Cannatrek shareholders may see their stake increase to as much as 68.2%.
Importantly, these CV shares are non-tradeable and non-voting until conversion.
Leadership and Governance: A Diplomatic Split

Little Green Pharma CEO, Paul Long
The leadership transition appears smooth, with a blended team drawing on both camps. Brent Dennison will become Chair, Paul Long takes the reins as Group CEO, while Cannatrek’s current CEO Jason Rance will head Australian operations. CFO duties go to Cannatrek’s Paula Butler, and LGP’s Alistair Warren continues as Company Secretary and Legal Counsel.
The board will consist of five directors: three from Cannatrek and two from LGP. Both companies will operate independently until the scheme is implemented on or around 1 May 2026.
Shareholder Support and Key Dates
The scheme already has board-level support from both sides. Cannatrek’s directors - who collectively control 22% of shares - have pledged their votes in favour, and the LGP board (13.1% of shares) is also on board.
A shareholder vote is slated for 2 April 2026, with final court approval targeted for 21 April. If all goes to plan, LGP will implement the merger on 1 May 2026.
Verdict: Bold, Risky, but Timely
While the CV shares provide a degree of downside protection, the real litmus test will be in execution. Merging two cannabis companies is not for the faint-hearted - and the industry is littered with stories of synergies lost in translation.
Still, both LGP and Cannatrek have proven they can integrate acquisitions (Cannatrek with Heyday, and LGP with HHI), and the proposed merger gives them the heft to stay competitive in increasingly mature - and unforgiving - medicinal cannabis markets.
For now, shareholders on both sides may be justified in sparking one up - to celebrate, of course.
READ ARTICLE
Latest News
ALL NEWS
Latest Web & Podcasts
ALL WEB & PODCASTS

Book Now for the Next Edition
Reach real investors through TechInvest Magazine, distributed as an independent insert in The Australian Financial Review, online via www.techinvest.online as well as across investor-focused social media channels.
Book now for TechInvest Edition 4, coming in 2024 with many opportunities to showcase your Tech insights.
What you get:
- Article on your company written by a highly experienced writer or supplied by your company
- Approved article included in TechInvest and replicated on www.techinvest.online
- Two page ‘Company in Focus’ features receive prominent pointer on front page of magazine
- Copy of article (copyright free) for you to use for own marketing purposes
- 10 copies of magazine

Upcoming Webinars
ALL WEBINARS











